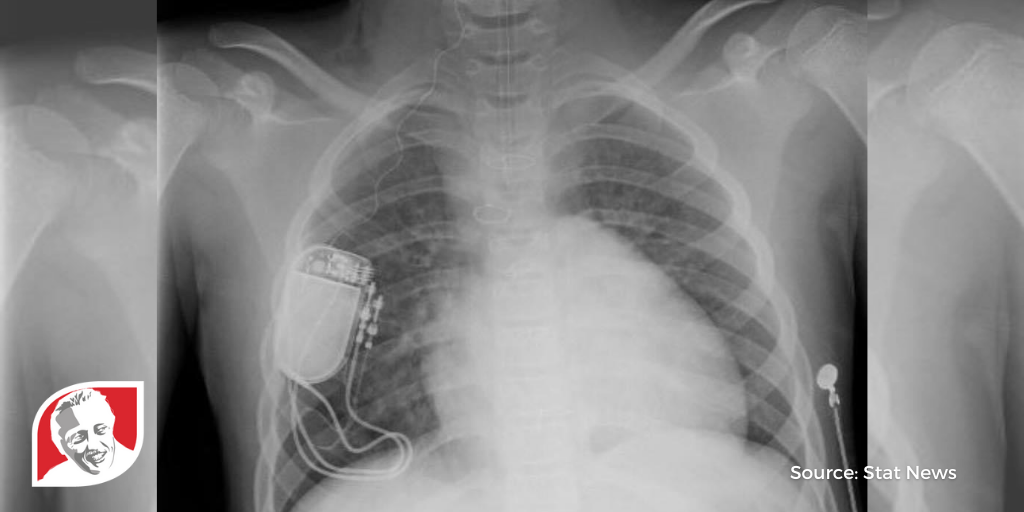
After six years of research and over 40 patient surgeries, doctors have successfully finished Phase I of trials for a new device that is helping children with Down syndrome sleep through the night.
Surgically implanted in the chest with a wire connecting to the tongue, Inspire Medical Systems’ hypoglossal nerve stimulator can be turned on via a remote control to move patients’ tongues while they sleep – helping keep their airways open.
The Phase I results of the trial published last week are very promising. According to the findings, only five of the 42 children with Down syndrome who received implants experienced mouth discomfort after the device was implanted.
To understand how the device is impacting patients, researchers analyzed their breathing patterns before and after surgery:
”[They] tracked the change in apnea-hypopnea index, or the number of times per hour when someone isn’t receiving a sufficient amount of oxygen while asleep. On average, the device helped patients to have about 50% fewer of these events per hour. For three-quarters of patients, the number of events dropped under the threshold for severe sleep apnea, which is 10 episodes or more per night.”
According to patient testimonials, the device has been life-changing. Kate Dougherty, whose son Elliot was one of the lucky trial members, recently shared:
“It’s been a miracle… Once we were able to get the sleep apnea under control, all of the other pieces began to fall together. I just can’t imagine how much better life would have been for Elliot if we could have done this sooner.”
The device already received FDA approval for use in adult patients in 2020, and the team at Inspire is working to get it approved for pediatric patients with and without Down syndrome. Not only does the implant help with sleep apnea, but evidence also suggests that the device could help “improve speech [in] patient[s] with Down syndrome” – providing rich potential for further research.





